Food Safety
IDFA Webinar: 3 Key Takeaways for Onboarding Rapid Spoilage Methods
Recently we hosted a webinar on what it takes to onboard a rapid spoilage method for finished product release with participation from FDA regulatory experts, process authority and major dairy producer. Below are the key takeaways from the presentation.
Takeaway 1 - QA Manager from Major Dairy Producer take on Rapid Spoilage Methods
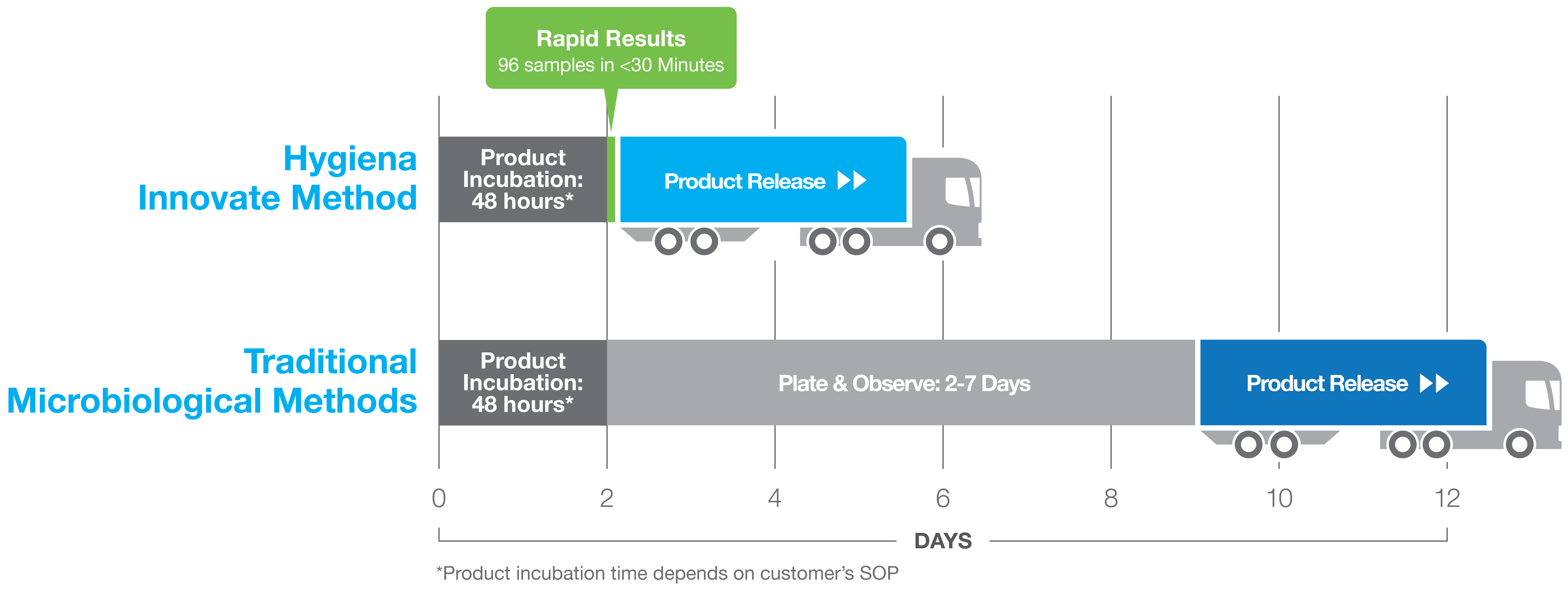
Innovate System can save dairy and juice manufacturers days for time-to-results when compared to traditional methods. This time savings directly results to cost savings potential through the reduction of warehouse space and pushing the product out to market faster.
Historically, finished aseptic dairy and beverage products have been tested utilizing traditional agar and pH methods, but within the past 20 years rapid methods have made an appearance and are widely adopted for the detection and release of finished aseptic dairy and beverage products. When we look at the reasons why, concerns with data integrity, time to result, and overall operational costs are key indicators of this trend. Bonnie Tang, QA Manager from Shamrock Food confirms her experience with onboarding rapid methods at her production facility.
Watch below:
Takeaway 2 - Process Authority Role
Process Authorities do not have the role of recommending which test method to use for product quality. Rather, they are tasked with confirming the method selected by the manufacturer to test their finished products is viable and robust. In the clip below, Pablo Coronel, PhD from CBR Group, represents process authority and its role.
Takeaway 3 - FDA Role in Rapid Method Onboarding
There are limited FDA regulations when it comes to testing finished aseptic products. This means it is solely up to the manufacturer to provide a robust product quality program, including product verification testing using traditional or rapid methods. We hear directly from FDA's regulatory expert Dan Geffin:
When it comes to confirming the quality of your product, consider the Innovate System for its operational efficiencies, ease of use, and access to industry-leading technical and scientific support. Not to mention the significant cost savings potential! To learn more, visit our website at hygiena.com/instruments-and-automation/monitoring-systems/innovate.