Food Safety
Everything You Need to Know About the FSIS Regulatory Framework for Poultry
Don’t have time to read the full blog? Check out the video!
Framework Recap
We will explain the three (3) components of the framework and highlight solutions to fulfill the component requirements to help the poultry industry develop food safety programs, evaluate their processes from flock to final product, and manage the data using online statistical process control tools from Hygiena.
FSIS has had a goal of reducing Salmonella infections linked to poultry products for some time; the current approach has not resulted in an acceptable level of success - 2010 and 2020 Healthy People targets were not met. The Healthy People 2030 target is to reduce Salmonella infections to a national case rate of 25% from all sources AND FSIS has adopted the same target of a 25% reduction in infections linked to FSIS-regulated products.
“Despite FSIS sampling data showing reductions in Salmonella contamination in poultry products, the current approach to Salmonella has not led to a demonstrable reduction in Salmonella infections.” -FSIS Leadership, 2022
During the five-year period from 2017 to 2021, the number of chicken samples in which FSIS detected Salmonella decreased by more than 50%. However, the estimated rate of human Salmonella infections from all sources has remained consistent over the last two decades.
Component 1
The first component of the framework requires incoming flocks to be tested for Salmonella before entering an establishment. This component aims to incentivize the use of preharvest interventions that reduce the level of incoming Salmonella contamination or mitigate the risk of a particular serotype entering the establishment.
- Flocks would have to meet a predetermined target for Salmonella at receiving.
- The target could be Industry-wide or Establishment-specific.
- Each flock received is accompanied by documentation demonstrating that the birds were tested for Salmonella before slaughter.
- Documenting the Salmonella levels or serotypes.
- FSIS would use periodic Salmonella testing at rehang to verify this documentation.
- Demonstrate subsequent processes that will effectively reduce Salmonella so that the final product standards will be met.
- If the flock does not meet the predetermined target at receiving, then the establishment could implement corrective actions and additional interventions.
- Example: processing a more contaminated flock at the end of the day (“logistical slaughter”).
- This assessment could be based on the documented ability of an establishment’s processes to decrease Salmonella consistently.
- If the flock does not meet the predetermined target at receiving, then the establishment could implement corrective actions and additional interventions.
Component 2
This component builds on HACCP and a prevention-based approach to food safety by enhancing Establishment Process Control Monitoring and FSIS Verification to ensure pathogen control throughout slaughter and processing operations. This component aims to enhance monitoring procedures, including revised locations for multipoint sampling and using a statistical approach to process control.
- FSIS may modify the existing requirements for indicator organism testing for process control.
- Establish additional parameters to better define the required analysis of the data.
- Establishments may be required to test for indicator organisms using APC or EB, with additional locations required for process control.
- Require a pre-chill location such as rehang and continue with the already existing sample location requirement of post-chill.
- Standardize the paired microbial data generated by establishments.
- Enables FSIS to improve instructions to FSIS in-plant personnel on how to verify establishments’ process control.
- FSIS would consider production volume when determining the frequency establishments must use for sample collection.
Component 3
Component 3 assesses whether certain levels or types of Salmonella on raw poultry product present an elevated risk of causing human illness and therefore, should be considered adulterants. As a result, the Agency is considering implementing a final product standard or standards to ensure that products contaminated with elevated levels of Salmonella or highly virulent Salmonella is not sold to consumers. This goal would be accomplished by declaring Salmonella an adulterant.
This would cause a severe disruption of commerce for Salmonella if declared as adulterated in poultry. This is a statement by the agency to incentivize the industry to make critical improvements.
In doing so, FSIS would rely on criteria that were applied to STECs:
- Consideration of serotypes associated with human illness
- Lowest infectious dose
- Severity of human illnesses
- Typical consumer cooking practices
FSIS has already released that Salmonella meets the criteria to be considered an adulterant in not-ready-to-eat (NRTE) breaded and stuffed raw chicken products based on limits or threshold approach (We have quantification and limits for these products available!). FSIS is exploring whether a single product standard for Salmonella may be appropriate in all raw poultry products.
- In developing a final product standard, FSIS would evaluate existing scientific support, stakeholder feedback, and access to test results to support timely identification of Salmonella serotypes or pathogenicity factors.
- The agency is considering initially developing an enforceable final product standard based on quantification rather than a “zero-tolerance” standard for Salmonella.
- This final product standard would promote Salmonella reduction by establishments and incentivize upstream practices that reduce Salmonella.
- The interventions at slaughter and processing must demonstrate that they can achieve the final product standard.
- If an enforceable final product standard is ultimately finalized and implemented, FSIS is considering sunsetting the current Salmonella performance standards, including the moving window approach to sampling and categorization of establishments.
Get the Hygiena Solutions
Now that we have explained the framework, components, and requirements, the industry is seeking tools and methods to support its efforts to fulfill the proposed regulations.
Let's walk through Hygiena's solutions for Salmonella Quantification and limits testing, indicator organism rapid detection and quantification tools, and a robust solution for data management and risk assessment. Salmonella Quantification and Limits Testing Hygiena has pioneered quantification with the innovation of SalQuant®. SalQuant has evolved over the past two years by focusing on direct industry needs, requirements, and feedback.
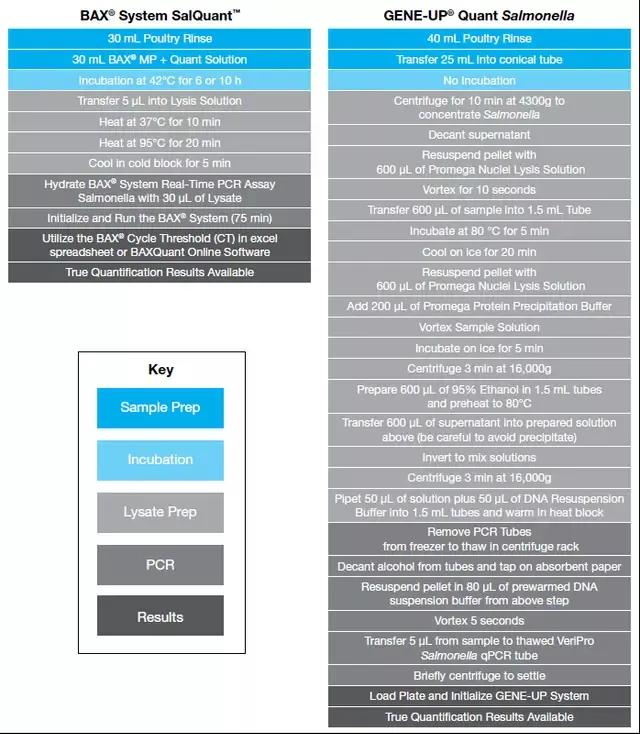
SalQuant® is applicable across the poultry production chain to handle highly contaminated samples that can be found in the primary production environment, such as boot swabs and dust swabs, throughout processing with single rinsate protocols to fulfill all sample locations from rehanging to post-chill and final products such as parts, ground poultry and the newly regulated, breaded, raw and stuffed chicken products. Check out our Quantification Manual to view + 20 Protocols for the Poultry Industry! With the framework components identifying and requiring an increase in testing volumes across a wide variety of sample types, we listened to the industry feedback for the need for ease of use, repeatability, and robustness in results. As you can see below, we have ensured that the BAX® Quantification and Limits methods are simple, require fewer steps and are easily repeatable by any technician to ensure the accuracy of results.
Indicator Organism Testing
Ease of use is essential, but we can't forget about time to results of all targets being tested. Now that pathogen quantification is achievable with same-day results; indicator testing shouldn't be the bottleneck to making release decisions or corrective actions. Hygiena’s MicroSnap® technology can provide results in less than 7 to 8 hours for APC's and EB's. That is at minimum, 16 hours faster than Petrifilm™. This solution also has the added benefit of data management at your fingertips since it utilizes the EnSURE® Touch luminometer with instant data uploads into SureTrend®.
Managing data allows analytics to be displayed in figures and dashboards to visualize the full process.
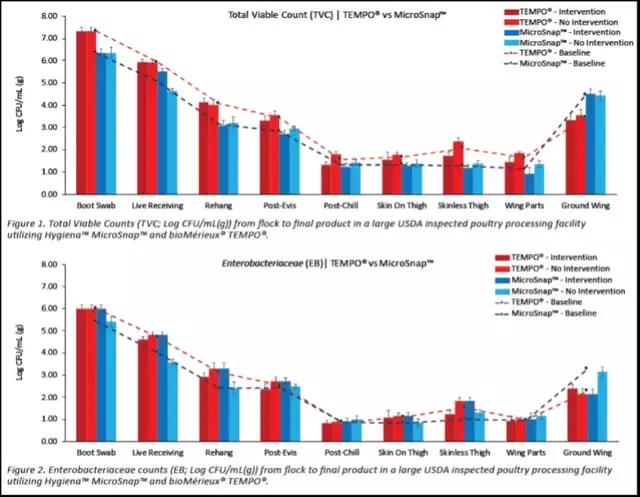
In a recent study using the MicroSnap technology, the data below was generated using the MicroSnap EB and TVC or APC test comparing interventions.
- This project also compared the MicroSnap technology to Tempo® with factors such as accuracy of results, sample processing, incubations, and result interpretation.
- Overall, results were available from the MicroSnap in 8 - 9 hours compared to Tempo 27 - 51 hours.
- This further highlights the need to pair up time to the results of pathogens and indicator organisms to manage process control and risk for decision-making.
View the full technical bulletin!
Data Management and Risk Assessment
FSIS heavily highlighted the need for standardized data management; however, the agency and the industry are still working toward what this could look like. So far, everyone has a different approach. Hygiena has created and updated our data management solution to integrate ATP, PCR, Indicator testing, and allergen data into a single location. When we pull all the FSIS framework components together, the ultimate goal is to support a system where we can:
- Visualize complex food safety test data & gain process intelligence.
- Access robust quantification and prevalence data that is always current and available.
- View trends and implement early interventions to reduce the risk of Salmonella in poultry products to consumers.
Check out all of the other benefits SureTrend® can provide!
We are so excited to share the Hygiena solutions with you - SalQuant and SalLimits™, QuantOnline™, MicroSnap®, SureTrend® for biomapping and trend Reporting, and all the data resources that have been created from over 100,000 data points across our applications and R&D teams, industry partners, and academic collaborators.
Speak with a team member today to get the information you need to start building your food safety program! Let's Chat