Food Safety
Explained by Experts: Top 5 Tips for Quantification You Need to Know
Welcome to the first series "Explained by Experts" with MDx Global Product Manager, April Englishbey.
Let’s jump right in and learn about quantification.
“I've been on board for quantification and commercially launched the first quantification method (or kit) being SalQuant™, and have been along for the whole ride. So, I wanted to share some experiences and attributes as a customer, what you should be looking for to ask your vendor about quantification.
As someone with hands-on experience developing quantification for the industry, I wanted to share the critical components we consider when we start the development of any type of quantification, as well as how it works for the industry to ultimately fulfill the goal of reducing consumer risk.”
Tip 1
“Number one is the flexibility of data. With the data you're looking to capture and use, think about what do you actually want to know? When you get a result of 5.7 CFU per/g, how do you actually use that as a decision-making tool? This is some of the consultative discovery that we go through with customers to get to the root of how you need to utilize the additional information.”
Knowing the answer to these questions helps you decide which of the two buckets of quantification you need based on the flexibility and purpose of the data.
- True quantification, like SalQuant, utilizes an identified timepoint of incubation coupled with software to determine the number of bacteria in the original sample.
- Limits testing simply identifies when a positive occurs at a specified time point, indicating that the number of bacteria is greater than the threshold that has been set. Sometimes this style of testing is also known as semi-quant, but limits and threshold testing are a little bit less confusing.
Really understanding what type of data you're looking for and how you would make a decision using that data is really important! At the end of the day, does the method you’re using have the flexibility to utilize a single sample or protocol to perform either quantification or limits testing?
Tip 2
“The second tool is dynamic matrix options.
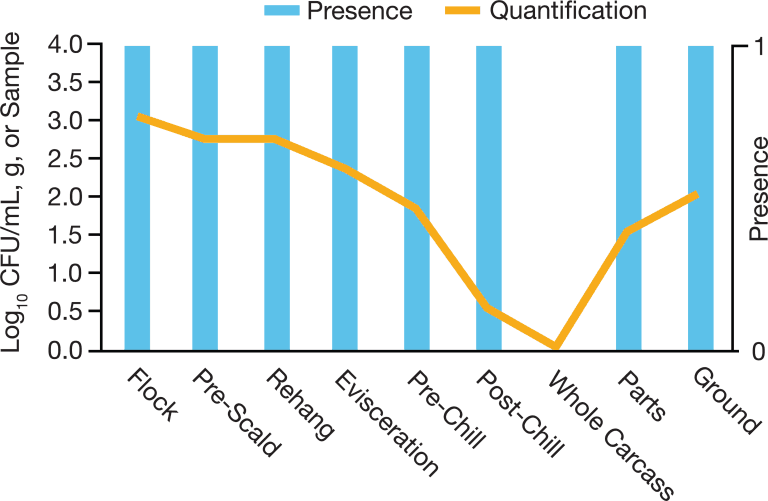
Food safety is not just final products, the majority of times those samples do get the biggest focus. However, processing and pre-harvest are part of the equation also to be concerned about to manage the whole realm of food safety.
When we started this journey 3 years ago we first developed quantification for final products and gathered great data, BUT we still needed to figure out why the bacteria still surviving after all of the interventions applied throughout the process.
If we can clean up on the front end of animal and food production we have a greater chance of removing Salmonella throughout the process and reducing that risk. That's why we can see industries and influencers not only gathering data from the final product but adding consistent data points throughout their processes and truly evaluating if the interventions are working. We also continued to work backward to the farm or pre-harvest levels to measure where we can impact the load of Salmonella that comes in and what comes out.”
Looking for vendors that have the robustness in their method to work across varieties of matrices and even the dirty samples like rehang versus post-chill rinsates, both are still rinsates, but verifying the method works due to the nature of “dirtiness”.
Not all samples are created equal even though they may look similar so ensuring verifications have been performed is important to see the data yourself.
Tip 3
“Number three is the wider enumerable range.
Methods need to be able to get to the lower end (1 CFU) as well as the higher end (10,000 CFU) of the range of quantification because we need flexibility based on the sample type as well as when there is a critical failure that happens with bacteria flourishing out of control.”
Within food safety programs, we know that sometimes there will be an outlier (low or high) and the method needs to be able to quantify compared to reporting a negative or greater than enumerable range, which isn’t a helpful data point.
“I like to use the example of the rinsate, as I mentioned in tip 2, for process control to look from dirty to clean. Being able to quantify that pre-intervention there is a high number of bacteria, and then post-intervention there is still a positive but in a very low amount knowing that it's still detectable and quantifiable is important to truly measure the effectiveness of the process and interventions.”
It's really important to understand that the expectation from a final product is the cleanest and majority of the time void of pathogens. Results should not be at high levels like 100 CFU/g or mL. Even 10 CFU/g can be too high depending on the industry. Methods that have a lower end of only quantifying 100 CFU/g really aren't helpful in a final product because the user will have a false sense of “cleanliness” when quantification is seen as negative.
Tip 4
“The fourth tip is the process should be easy to use."
From a regulatory perspective, prevalence will always be a critical component to measure consumer risk, quantification is not meant to fully replace prevalence. It's more of a value add. When thinking about the process of needing to fulfill regulatory needs but also gather more information, utilizing the same sample throughout the process is really helpful for time and labor resources.
"Utilizing a single sample is essentially why we designed our protocols at Hygiena to have a streamlined workflow where the sample can be set up once to get both quantification results, that tell me if my sample is positive with a high risk, or alternatively it’s negative and need to continue to prevalence to manage the regulatory risk. The value of quantification is not meant to be disruptive into normal processes. If you're adding more resources and more cost or labor, is that really a value add at the end of the day? The data is still very valuable, but if it's so hard to gather because of the process it become prohibitive. We need to have dynamic data being numerical data versus by just the ones and zeros or the positive and negatives.”
If it's not as easy to do in the lab, industries and companies could fall back just to doing the minimum of prevalence, which is not where we need to go for food safety.
Tip 5
“The last tip is to see the data.”
For any method, proving success with data from validations or verifications is so important to ensure that the vendors and method developers have done their due diligence.
“The beauty of using PCR for quantification is that the assays have already been validated for the core component of detecting the pathogen at a 1 CFU/sample level for prevalence, which is more difficult than quantification. Since all the boxes have already been checked for inclusivity and exclusivity all we're doing is adding in quantification using already established tools for microbiology."
Not only should you see the data, but really understand the amount of data and how it was collected and compared. Only developing or validating using 10 samples is not enough, at minimum 30 samples creates statistical relevance and trust in the comparisons to reference methods, but that is an absolute minimum.
Let’s Wrap Up
The way we think about quantification and the reasons behind all these kind of questions and comments that you may hear in the industry and in government is make sure you have your own informed information and awareness.
“Utilizing a single sample is essentially why we designed our protocols at Hygiena to have a streamlined workflow where the sample can be set up once to get both quantification results, that tell me if my sample is positive with a high risk, or alternatively it’s negative and need to continue to prevalence to manage the regulatory risk. The value of quantification is not meant to be disruptive into normal processes. If you're adding more resources and more cost or labor, is that really a value add at the end of the day? The data is still very valuable, but if it's so hard to gather because of the process it become prohibitive. We need to have dynamic data being numerical data versus by just the ones and zeros or the positive and negatives.”
If it's not as easy to do in the lab, industries and companies could fall back just to doing the minimum of prevalence, which is not where we need to go for food safety.