Food Safety
What Does "Gluten-Free" Really Mean?
What does “Gluten-free” really mean?
The term “gluten-free” is covered by legislation worldwide.
In the US, it falls under the regulation of the FDA. They use an analytical approach to define "gluten-free" and have adopted <20 ppm as the criteria based on validated methods for gluten detection and scientific studies that this level of gluten can be tolerated by most individuals with celiac disease.
In the EU, it falls under Regulation (EU) 828/2014 for the labeling of gluten-free foods. Based on the Codex Alimentarius Standard for gluten-free, the law stipulates that only foods containing a maximum of 20 parts per million (ppm) gluten content or less can be labeled as “gluten-free”.
What is an appropriate test for the measurement of gluten in foods?
A scientific method used for product testing for gluten is the quantitative ELISA. If the test provides results < 20 ppm, the product can be claimed to be “gluten-free.”
In the US, the Gluten Intolerance Group (GIG) and the Gluten-Free Certification Organization (GFCO) recognize the feasibility of the G12 antibody, and the GlutenTox® Pro kit, for detection of gluten levels in food products. This is because the G12 antibody kits have AOAC-RIPTM approvals, and the FDA accepts any gluten test that is validated and AOAC certified.
In the EU, the G12 antibody is generally not recommended for testing. Only the R5 antibody is referenced by Codex Alimentarius and endorsed by the Association of European Coeliac Societies (AOECS).
Below is a quote from AOECS:
“For a rapid in-house control of raw materials and surfaces, as well as to check the effectiveness of cleaning procedures in production equipment, the lateral flow test based also on the R5-antibody can be used. If heat-treated materials are to be tested, the 'Cocktail' extraction must be used. In case of a positive result, the gluten concentration must be determined by ELISA.
* In the collaborative study for approval of the Codex Method the R5-antibody was used with the R5 ELISA RIDASCREEN® Gliadin R7001 test kit from R-Biopharm . If test kits from other companies based on the R5 antibody, but with extraction solutions other than the 'Cocktail' are used, it is recommended to compare the results with the RIDASCREEN® Gliadin R7001 .”
How would you interpret this information?
Hygiena® believes this quote is a bit misleading.
- It states that the R5 antibody can be used but doesn't mandate its use. This means that it is also permissible to use other antibody tests targeted against the gluten protein, gliadin (including the G12 antibody used in Hygiena® GlutenTox® kits).
- The statement “if heat-treated materials are to be tested, the 'Cocktail' extraction must be used” is outdated and requires the use of toxic compounds. Alternative less toxic and environmentally acceptable extraction solutions have been developed with equal or better performance than the Mendez cocktail (e.g., Grace et al 2012, AOAC paper) and many are AOAC approved for use.
- The statement “* In the collaborative study for approval of the Codex Method” is confusing as
- Codex does not approve methods.
- There is no certified reference material for gluten/gliadin.
- AOECS participates in the Codex Alimentarius Commission but is there as a representative of the gluten intolerant population and should not promote the same method for years, despite improvements and advancements in technology.
- Lastly, the mention of a specific test kit by AOECS and Codex is inappropriate since such groups should remain neutral and focus on the best technologies that provide the most accurate results and is in the best interest of gluten-sensitive populations. This means providing resources for all tests and technologies available for gluten testing.
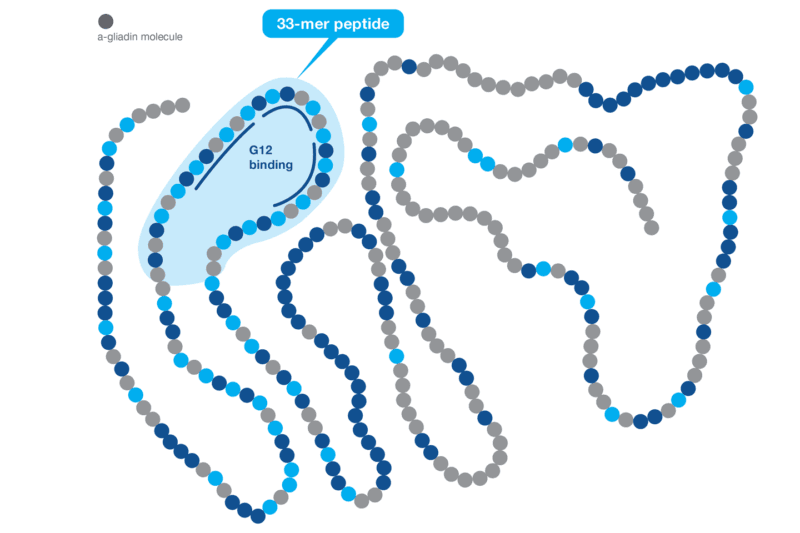
Harnessing the Power of the G12 Antibody Based on scientific data, Hygiena® has demonstrated that the G12 antibody is a better choice for gluten detection. First, the G12 antibody was raised against the highly immunogenic 33-mer peptide of the α-gliadin protein that induces celiac disease and recognizes the immunotoxic prolamins from wheat, barley, rye, and some varieties of oat. Second, the G12 antibody is also capable of reacting to other epitopes that are found in other toxic prolamins and can react to similar sequences present in other gliadins outside the 33-mer of the α-gliadin. A recent scientific study has shown that the G12 antibody recognizes the most immunogenic peptides responsible for 80-95% of the immunoreactivity of T cells in celiac patients.
Hygiena® has used the G12 antibody in the development of our gluten detection products. Our GlutenTox® ELISA kits are designed to quantitate the amount of gluten in food samples. Our GlutenTox® ELISA Rapid G12 can detect and quantify very low levels of gluten, down to 0.6 ppm, in 90 minutes from a wide variety of food matrices. Our GlutenTox® ELISA Competitive G12 has been validated for a wide range of hydrolyzed samples and complies with HACCP, ISO 22000, BRC and IFC guidelines.
Our GlutenTox® Sticks are designed for rapid gluten detection in food or drinks and on surfaces. The GlutenTox® Sticks Plus can detect down to 3 ppm (<20 ppm is considered “gluten-free”) and also fulfills HACCP, ISO 22000, BRC and IFS compliance. When paired with the Hygiena® Cube, qualitative, quantitative and semi-quantitative data can be collected and stored. The Cube has a detection range of 1 - 40 ppm of gluten and is validated for major food matrices. GlutenTox® Pro is also a quick test for gluten detection - it utilizes lateral flow technology and the G12 antibody and is ideal for environments with incoming ingredients and shared facilities and helps ensure the safety of the final food product (restaurant kitchens, catering companies and food manufacturers).
Learn more about our GlutenTox ELISA kits, our GlutenTox sticks, the Hygiena Cube, and our G12 antibody.